
Schematic illustration of enterokinasemediated cleavage. (A,B)... Download Scientific Diagram
The serine protease enterokinase is the physiological activator of trypsinogen and has a specificity for the sequence (Asp)4-Lys-Ile. The enzyme consists of two subunits linked by a disulfide bond. The heavy chain achors enterokinase in the intestinal brush border membrane and the light chain is the.

Enterokinase stimulates DNA synthesis in cells expressing EKTR... Download Scientific Diagram
Human enteropeptidase (hEP), also known as enterokinase, is an essential enzyme in food digestion and is localized at the brush border of the duodenal and jejunal mucosa 1,2,3.Activated hEP can.

Enterokinase cleavage and tags removal from the Hax1... Download Scientific Diagram
Enterokinase (enteropeptidase) is expressed only in proximal small intestine, where it initiates digestive enzyme activation by converting trypsinogen into trypsin. To investigate this restricted expression pattern, mouse enterokinase cDNA was cloned, and the distribution of enterokinase mRNA and enzymatic activity were determined in adult mice and during gestation. Analysis of enterokinase.

Apa Fungsi Enterokinase Safekey
Abstract. Enterokinase is a glycoprotein and is now designated enteropeptidase (E.C.3.4.4.8.). It is present in the duodenal and jejunal mucosa. Pancreatic proteolytic enzymes are secreted as proenzymes. Enterokinase converts trypsinogen to trypsin in the duodenal lumen.
.jpg_img_upload_solution_2022-09-05 11:10:53.987324.png)
What is the Enterokinase involved in?
A previously described patient developed celiac disease at age 25 years, but enterokinase levels remained low after normalization of intestinal mucosa with gluten-free diet. [ 2] Enterokinase, also known as enteropeptidase, is a key enzyme for intestinal digestion of proteins. Therefore, enterokinase deficiency causes severe protein.

Apa Fungsi Enterokinase Safekey
One of the most popular enzymes used for the in vitro cleavage of fusion proteins is enterokinase (EK, E.C. 3.4.21.9). EK cleaves with high specificity after the sequence Asp 4-Lys (DDDDK), which allows the fusion protein to preserve its native amino acid terminus without any additional unwanted cleavage residue from the recognition sequence.However, the complete removal of EK after protein.
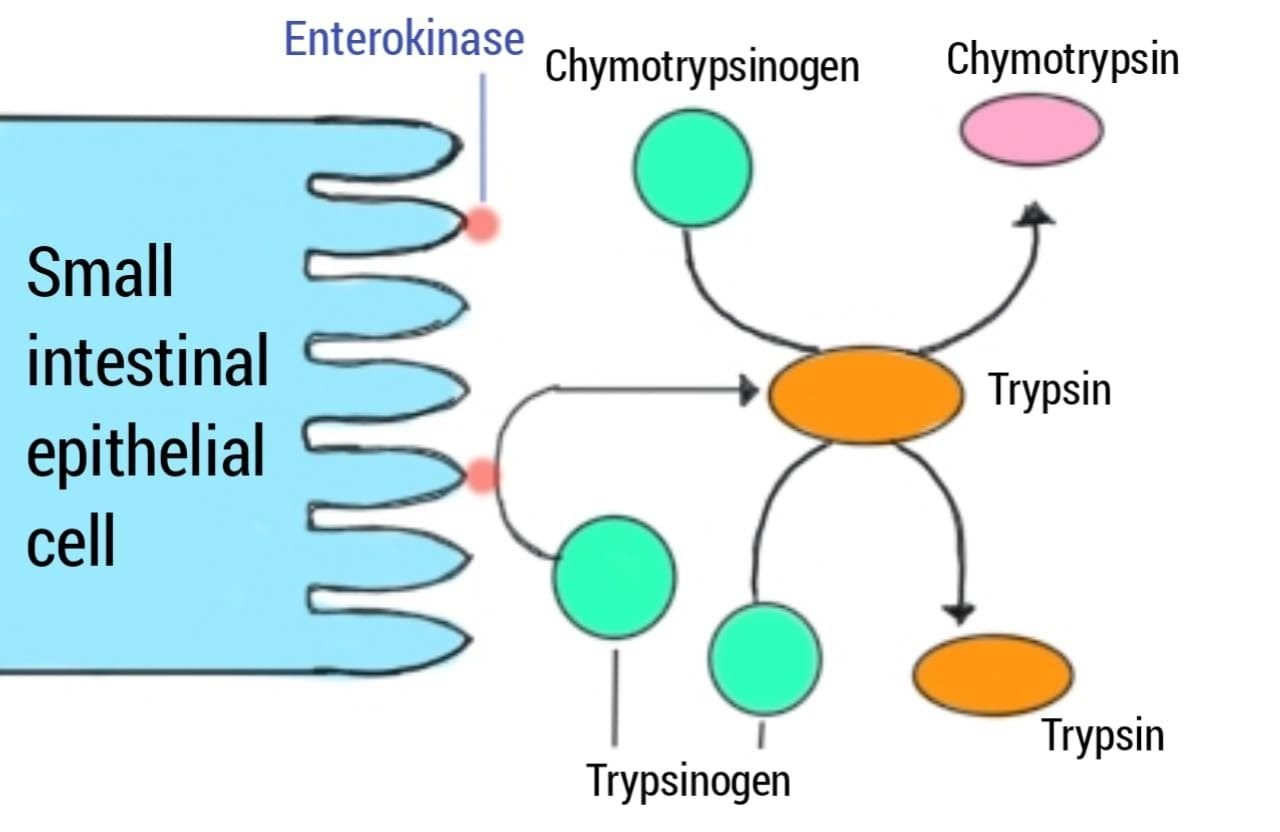
Is Enterokinase a brush border enzyme?
The role of enteropeptidase and trypsin in the process by which pancreatic proteolytic zymogens are converted into active enzymes has been investigated in the past, using purified enzymes and proenzymes of animal origin. In the present study, we wanted to study this process under conditions which come near to the physiological situation, which.

Enterokinase Protein Overview Sino Biological
Enzim enterokinase terdapat pada organ pencernaan manusia yaitu usus halus. Berdasarkan fungsinya bagian bagian usus halus membantu enzim lain sebagai aktivator enzim erepsinogen, enzim tripsinogen, pengubah sari-sari makanan dan pengubah pepton. Dari beberapa fungsi tersebut, tentunya anda penasaran bukan sebenarnya bagaimana enzim enterokinase bisa ada di dalam tubuh manusia.
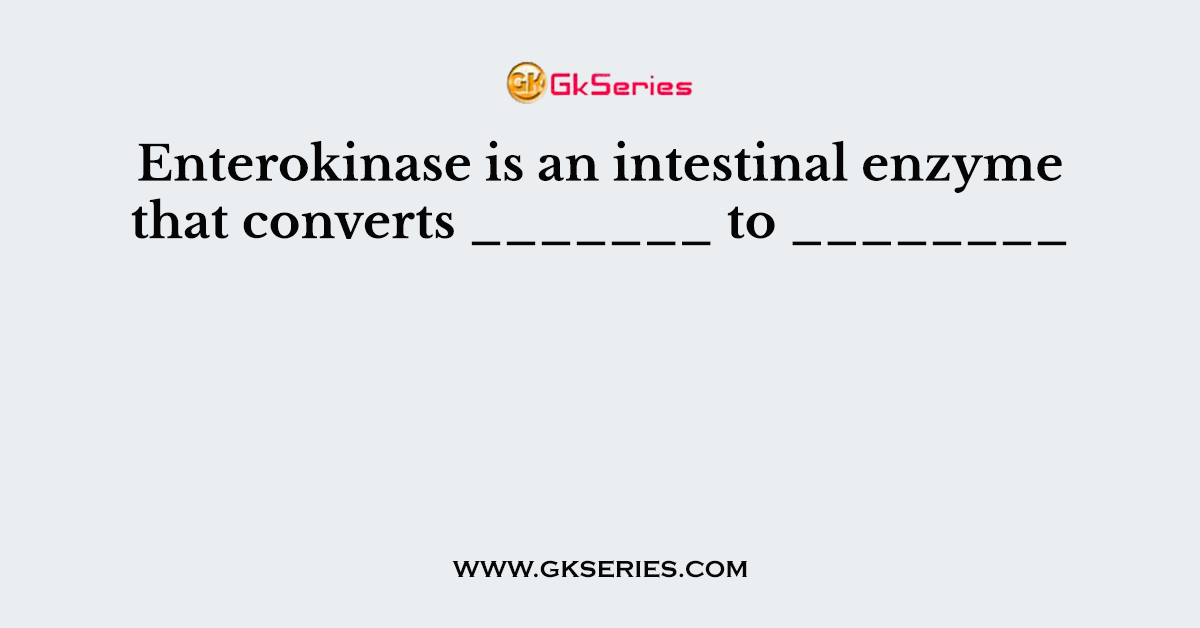
Enterokinase is an intestinal enzyme that converts _______ to
Enterokinase is an essential enzyme for normal mammalian digestion. The enzyme is the physiological activator of trypsinogen, and the trypsin produced activates other zymogens forming a mixture of proteolytic enzymes1. In the absence of enterokinase, intestinal digestion of foodstuffs is impaired. Newborn infants with a congenital deficiency of.

Enterokinase Protein Overview Sino Biological
Medical Care. Pancreatic enzyme replacement is indicated in patients with intestinal enterokinase deficiency. In exocrine pancreatic deficiency, the efficacy of enzyme substitution therapy appears to be higher when enzymes are administered either portioned along with meals or just after meals. Administration of enzymes in the form of enteric.

The enzyme enterokinase helps in the conversion of
Enterokinase is a protease of the intestinal brush border that specifically cleaves the acidic propeptide from trypsinogen to yield active trypsin. This cleavage initiates a cascade of proteolytic reactions leading to the activation of many pancreatic zymogens. The full-length cDNA sequence for bovine enterokinase and partial cDNA sequence for.

Apa Fungsi Enterokinase Safekey
Human enterokinase light chain (hEK L) specifically cleaves the sequence (Asp) 4-Lys↓X (D 4 K), making this a frequently used enzyme for site-specific cleavage of recombinant fusion proteins. However, hEK L production from Escherichia coli is limited due to intramolecular disulphide bonds. Here, we present strategies to obtain soluble and active hEK L from E. coli by expressing the hEK L.

Structure of murine enterokinase (enteropeptidase) and expression in small intestine during
Enteropeptidase (also called enterokinase) is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen (a zymogen) into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment.
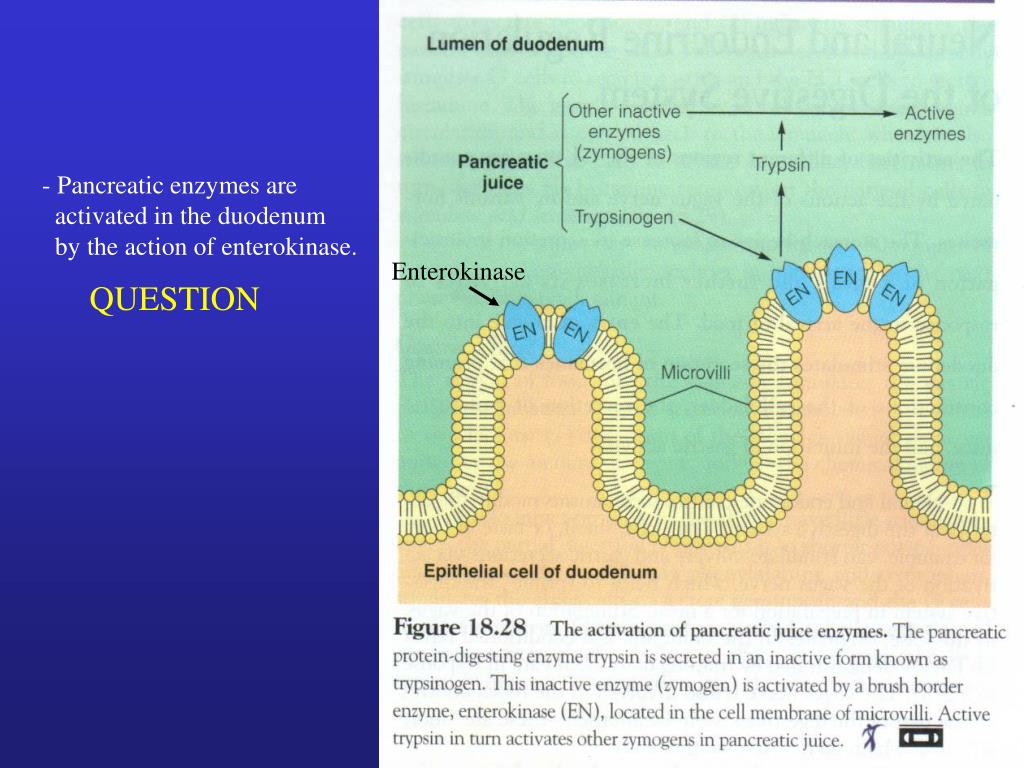
PPT Pancreas PowerPoint Presentation, free download ID6121477
Enterokinase (EK, also known as enteropeptidase) is a membrane-bound serine protease found in the duodenum and initiates activation of pancreatic hydrolases by cleaving and activating trypsinogen. Although the canonical target site for enterokinase is DDDDK, it is known that EK does not exhibit high stringency in its specificity for this.
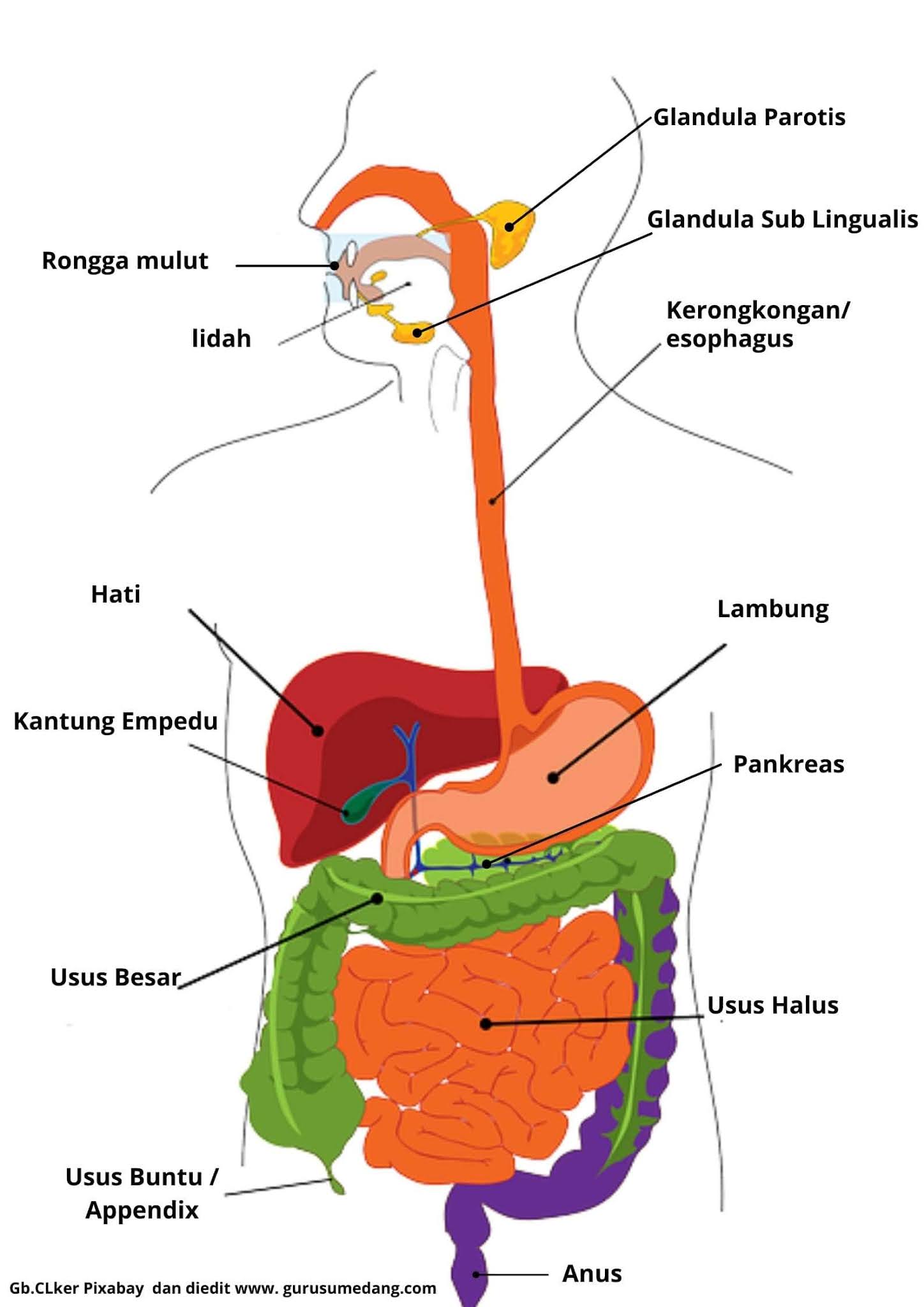
Sistem Pencernaan Makanan Pada Manusia GURU SUMEDANG
enterokinase, proteolytic enzyme ( q.v. ), secreted from the duodenal mucosa, that changes the inactive pancreatic secretion trypsinogen into trypsin, one of the enzymes that digest proteins. Enterokinase is believed to be produced by the glands of Brunner in the membrane lining of the duodenum. It resists destruction from the various enzymes.

Apa Fungsi Enterokinase Safekey
Enterokinase is a protease of the intestinal brush border that specifically cleaves the acidic propeptide from trypsinogen to yield active trypsin. This cleavage initiates a cascade of proteolytic reactions leading to the activation of many pancreatic zymogens. The full-length cDNA sequence for bovine enterokinase and partial cDNA sequence for.