
H2so4 Estrutura De Lewis
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

H2SO4 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2SO4 (sulfuric acid). In order to draw the lewis structure of H2SO4, first of all you have to find the total number of valence electrons present in the H2SO4 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
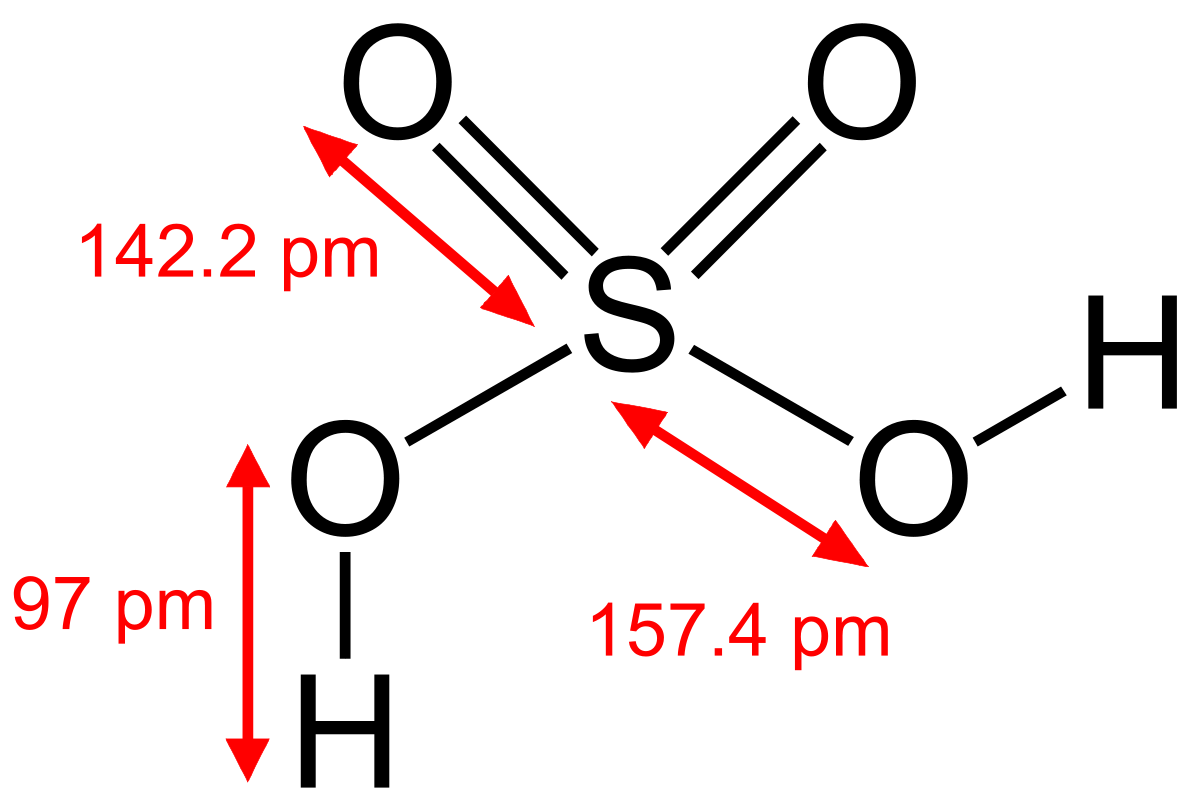
Sulfuric acid Wikipedia
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

H2SO4 Lewis Structure Sulfuric Acid YouTube
This chemistry video tutorial explains how to draw the lewis structure of H2SO4 - Sulfuric Acid.How To Draw Lewis Structures: https://www.youtube.
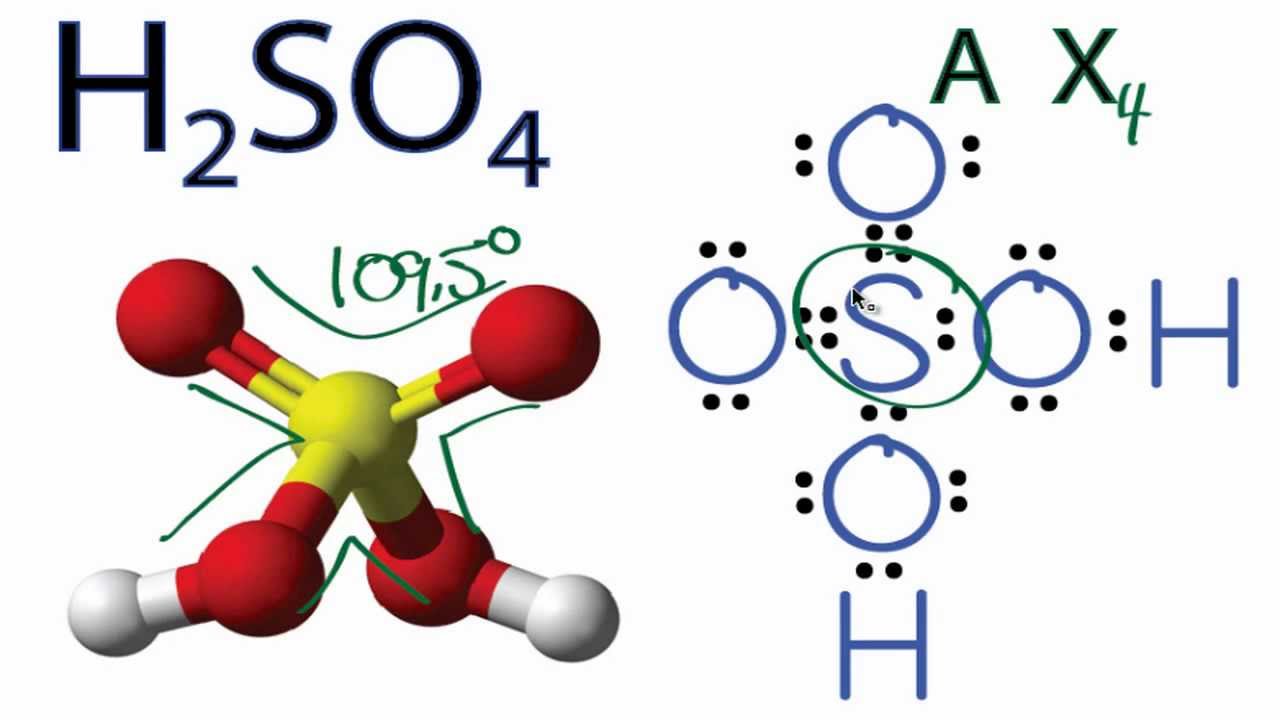
H2SO4 Molecular Geometry / Shape and Bond Angles YouTube
Steps of drawing H2SO4 lewis structure Step 1: Find the total valence electrons in H2SO4 molecule. In order to find the total valence electrons in H2SO4 (sulfuric acid) molecule, first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

H2so4 Estrutura De Lewis
Acid-base Reactions without Transferring Protons. The major utility of the Lewis definition is that it extends the concept of acids and bases beyond the realm of proton transfer reactions. The classic example is the reaction of boron trifluoride with ammonia to form an adduct: BF3 +NH3 → F3B−NH3 (10.5.1) (10.5.1) BF 3 + NH 3 → F 3 B − NH 3.
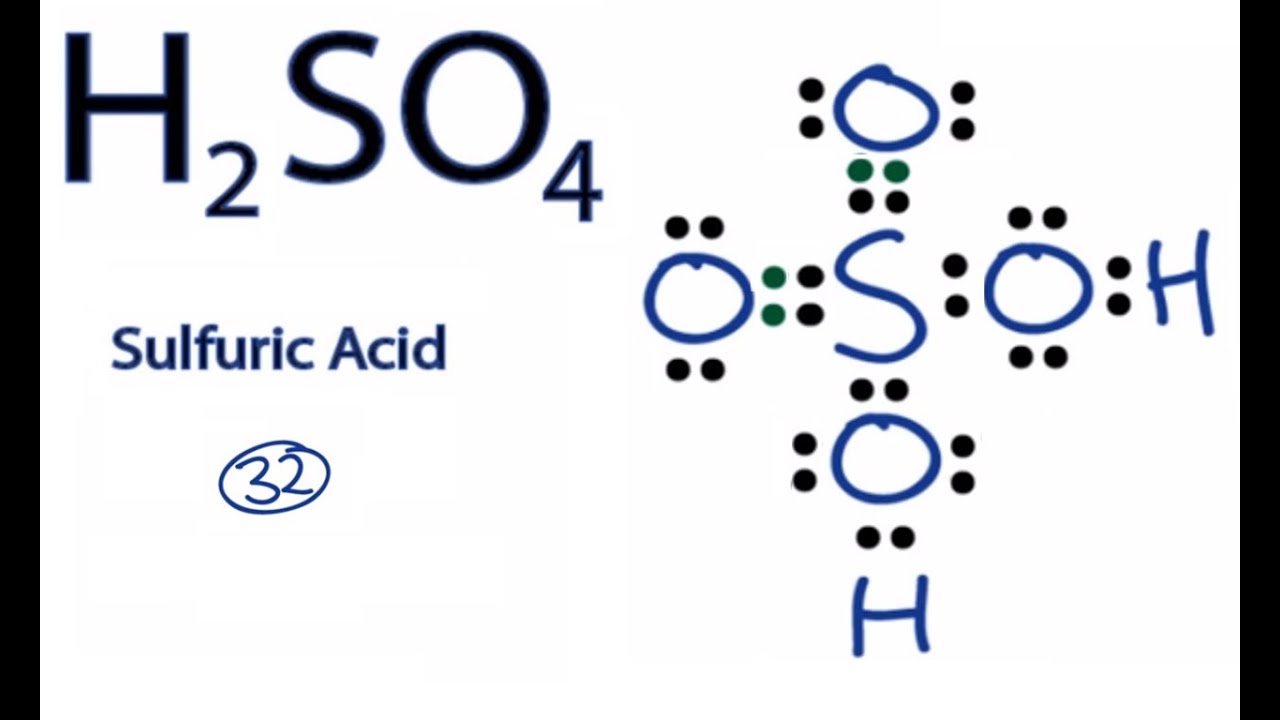
lewis dot structure for h2so4
Sulfur trioxide (SO3) is generally a colorless liquid. It can also exist as ice- or fiber-like crystals or as a gas. When SO3 is exposed to air, it rapidly takes up water and gives off white fumes. It can react with water to form sulfuric acid. SO3 is also called sulfuric oxide and sulfuric anhydride.
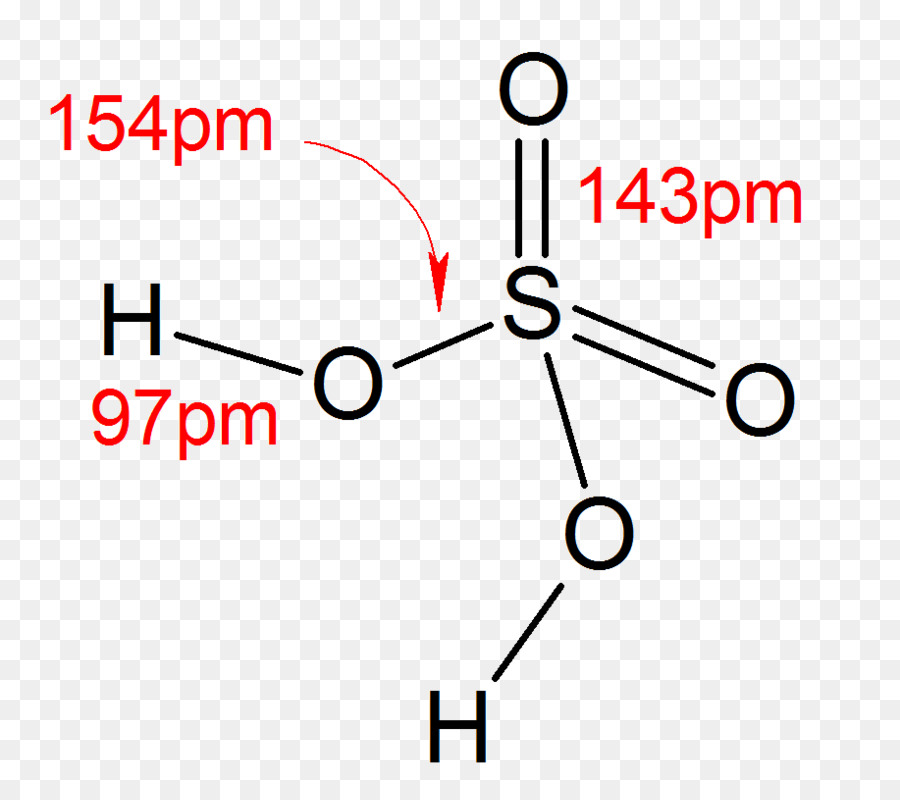
ácido Sulfúrico, Estrutura De Lewis, ácido png transparente grátis
I quickly take you through how to draw the Lewis Structure of H2SO4 (Sulfuric Acid). I also go over hybridization, shape and bond angles.

Draw the Lewis structure of sulfuric acid H2SO4 with minimized formal charges Include lone pairs
H2SO4 is a chemical formula of Sulfuric acid which is commonly known as Oil of Vitriol. It's a mineral acid composed of elements like oxygen, hydrogen, and sulfur. It has a molecular weight of 98.079 g/mol. H2SO4 works as an oxidizing and dehydrating agent. Furthermore, it's diprotic in nature which holds the capacity of releasing two.
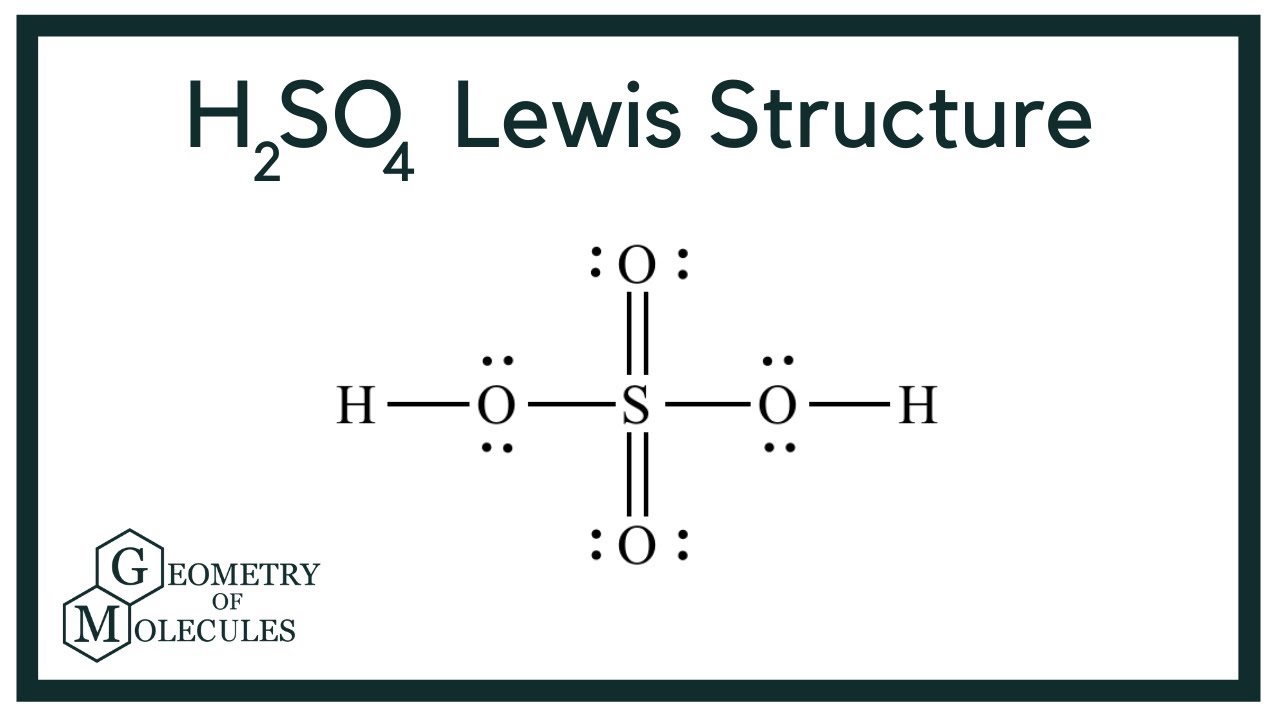
H2SO4 Lewis Structure (Sulfuric Acid) YouTube
878. 05:56. H2SO4 Lewis Structure - Sulfuric Acid. The Organic Chemistry Tutor. 626. Select textbook and university. Improve your experience by picking them. 1. Intro to General Chemistry 3h 53m.

QUIMICA Ácido Sulfúrico H2SO4 Hibridación enlaces sigma y pi AULAEXPRESS YouTube
your instructions for only $21/task. Learn more. Sulfuric acid, with the chemical formula H2SO4, consists of two hydrogen atoms (H), one sulfur atom (S), and four oxygen atoms (O). The Lewis structure of H2SO4 allows us to visualize the arrangement of atoms, the bonding patterns, and the distribution of valence electrons within the molecule.
Métodos para Acido Sulfurico Estructura De Lewis Paso a paso La fisica y quimica
Here's how you can easily draw the H 2 SO 4 Lewis structure step by step: #1 Draw a rough skeleton structure. #2 Mention lone pairs on the atoms. #3 If needed, mention formal charges on the atoms. #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure.

Estructura De Lewis Del Ácido Sulfúrico (H2SO4) YouTube
The Lewis Acid accepts the electrons from the Lewis Base which donates the electrons. Another case where Lewis acid-base theory can explain the resulting compound is the reaction of ammonia with Zn 2 +. \[ Zn^{2+} + 4NH_3 \rightarrow [Zn(NH_3)_4]^{4+} \label{2}\] Similarly, the Lewis Acid is the zinc Ion and the Lewis Base is NH 3. Note how.

QUIMICA Resonancia del ácido sulfúrico H2SO4 Lewis Carga formal AULAEXPRESS YouTube
H2SO4 Lewis Structure. Step 1 - in the first step, we should count the valence electrons for the H2SO4 lewis structure. In the H2SO4 lewis structure, there are three types of atoms S, O, and H present. Now S is the group 16 th element and belongs to the O family, so it has six electrons in the valence shell for S.

QUÍMICA ESTRUCTURA DE LEWIS Ácido Sulfúrico H2SO4Carga Formal Octeto Expandido AULAEXPRESS
For H2SO4 we have a total of 32 valence electrons. Put a pair between the atoms. We're forming chemical bonds here. We've used 8, 10, 12, and then around the outside to fill the octets on the Oxygens, 14, and 32. So this looks like a pretty good Lewis structure for H2SO4. We've used all 32 valence electrons and each of the atoms has a full.
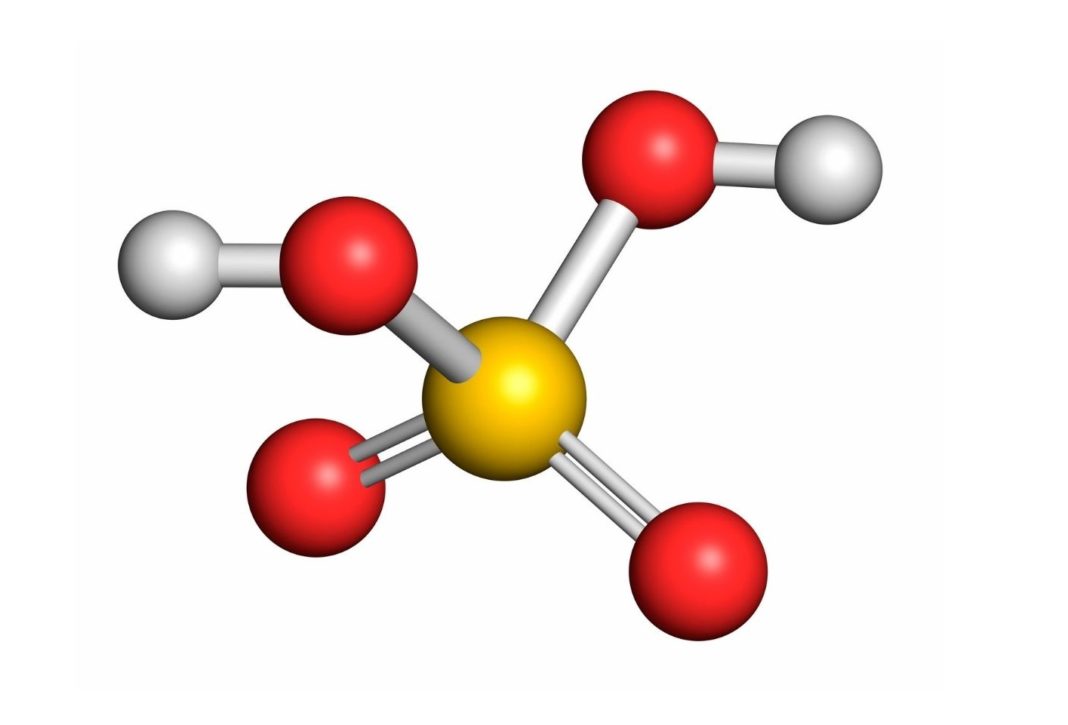
Ácido sulfúrico (H2SO4) estructura, propiedades, obtención, aplicaciones
Lewis structure of sulfuric acid is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of H2SO4.Sulfur is the central atom in H2SO4.