
Nitrogen Gas, For In Laboratory at Rs 400/cubic meter in Bengaluru ID 15678722333
The second method is to us Avogadro's Volume at STP 22.4L = 1mol. In both cases the significant digit count is three figures which means the acceptable solution is 125L. I hope this was helpful. There are two ways to calculate this if we assume STP of 1 atm and 273 K for the pressure and temperature. We can use the Ideal Gas Law equations PV.
NITROGEN COMPRESSED GAS CYLINDER 10L(incl.N2 gas) Shopee Malaysia
Jadi, massa dari 10 liter gas nitrogen (N2) pada 27°C, 2 atm adalah 22,764 gram. Pada suhu dan tekanan di luar keadaan standar atau keadaan kamar, volume gas dapat ditentukan dengan persamaan gas ideal. Rumus: PV = nRT. n n N2 m Mr N2 Massa N2 = = = = = = = = = = RTPV 0,082 L atm mol−1K−1x (273+27) K2 atm x 10 L 0,082 L atm mol−1K−1x.

Nitrogen Moregas
The calculator below can be used to estimate the density and specific weight of gaseous nitrogen at given temperature and pressure. The output density is given as kg/m 3 , lb/ft 3 , lb/gal (US liq) and sl/ft 3 . Specific weight is given as N/m 3 and lb f / ft 3 . Temperature. Choose the actual unit of temperature: °C °F K °R.

Nitrogen Gases, नाइट्रोजेन गैस in Noida , Nova Industrial Gases ID 8551831133
Active mass : It is defined as the number of moles present in one liter. Or we can say that the concentration of a reacting substance expressed in moles per liter. As we know that, At N T P, the 1 mole of gas contains 2 2. 4 liter volume of gas. As, 2 2. 4 L volume of nitrogen gas present in 1 mole of nitrogen gas

Spesifikasi Tabung Gas Nitrogen
To find any of these values, simply enter the other ones into the ideal gas law calculator. For example, if you want to calculate the volume of 40 moles of a gas under a pressure of 1013 hPa and at a temperature of 250 K, the result will be equal to: V = nRT/p = 40 × 8.31446261815324 × 250 / 101300 = 0.82 m³.

MSA Cal Gas Nitrogen Dioxide 10 PPM In N2 58L 808977 Newcastle Safety Servicing
Nitrogen Weight and Volume Equivalents. Weight of Liquid or Gas. Volume of Liquid at Normal Boiling Point. Volume of Gas at 70°F (21°C) and 1 atm. lb.
/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)
Cara Menghitung Massa Jenis Gas
Beranda. Massa 5 liter nitrogen pada T dan P tertentu adala. Iklan. Pertanyaan. Massa 5 liter nitrogen pada T dan P tertentu adalah 5,6 gram. Berapa jumlah atom He yang terdapat dalam 10 liter gas He pada T dan P tersebut? (tetapan Avogadro = 6×1023; Ar N = 14) 1,2× 1023 atom. 2,4× 1023 atom. 2,7× 1023 atom.
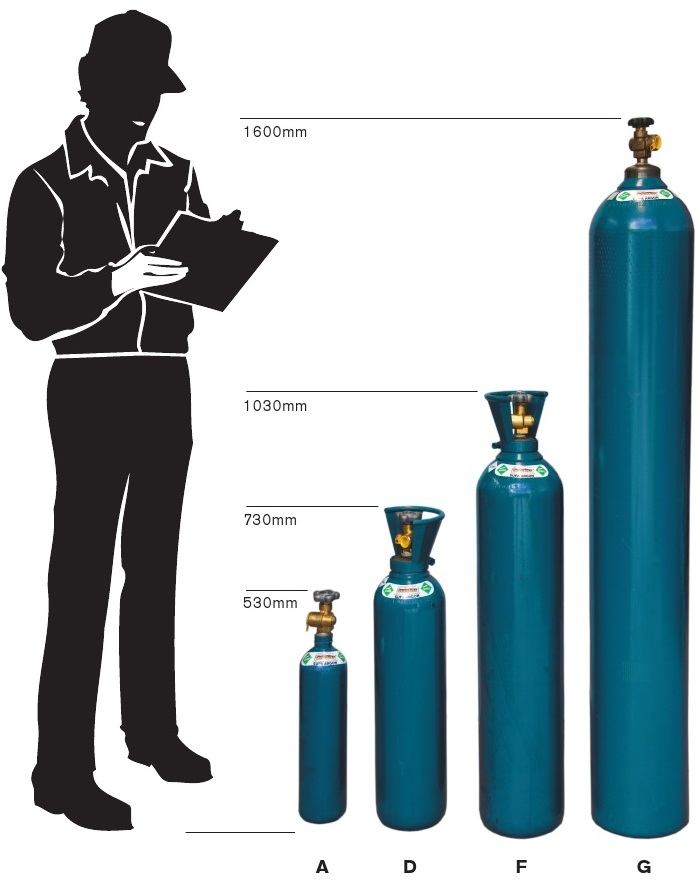
Nitrogen Gas Boc Nitrogen Gas Bottle Sizes
What is Nitrogen? Nitrogen (N 2) is a versatile gas used across a wide range of applications and industries, and we're always ready to supply it for new applications that arise.We offer nitrogen in multiple purities and supply modes. Purchase High-pressure Nitrogen Gas or Liquid Nitrogen. We offer compressed and liquid nitrogen in various grades specific to different applications, such as:

Jual Gas Nitrogen, Jual Gas Nitrogen di Bandung, Jual Gas Nitrogen di Medan, Jual Nitrogen Murah
Nitrogen Quantity Conversions Calculator. Scf (standard cubic foot) gas measured at 1 atmosphere and 70°F. Nm3 (normal cubic meter) gas measured at 1 atmosphere and 0°C. Liquid measured at 1 atmosphere and boiling temperature. Nitrogen quantity units converter. Supplier of new and used industrial gas plants and plant components plus related.

How and Why Is Nitrogen Gas Used For Packaging?
About Nitrogen gas; Nitrogen gas weighs 0.001251 gram per cubic centimeter or 1.251 kilogram per cubic meter, i.e. density of nitrogen gas is equal to 1.251 kg/m³; at 0°C (32°F or 273.15K) at standard atmospheric pressure.In Imperial or US customary measurement system, the density is equal to 0.0781 pound per cubic foot [lb/ft³], or 0.00072312 ounce per cubic inch [oz/inch³] .

Nitrogen Gas Phnom Penh Gas Center
This online calculator converts grams to liters and liters to grams given a gas formula. It uses molar volume of a gas at STP (standard temperature and pressure) This calculator finishes the topic started in Convert moles to liters and liters to moles calculator. Because the molar volume is the same for all ideal gases and is known, we can.

Jual Tabung Gas Nitrogen 1m3 di Lapak Sentra Gas Industri Bukalapak
Calculate pressure, volume, quantity (moles) or temperature of a gas with this versatile Ideal Gas Laws calculator (moles) by entering the other three. Free online gas law calculator a.k.a. PV = nRT calculator which accepts different input metric units such as temperature in celsius, fahrenheit, kelvin; pressure in pascals, bars, atmospheres; volume in both metric and imperial units cubed.

Jual Gas Nitrogen UHP, N2, Jual Gas Nitrogen Food Grade, Jual N2, Jual Nitrogen N2, Harga
VIDEO ANSWER: So in this problem we want to find a mass of nitrogen dioxide gas given these conditions. This is uh and basic employment of the ideal gas law. And it is probably easier if you rearrange the constants to get in which is the mold of. Berapa massa dari 5 liter gas nitrogen ( N2, Mr=28) pada 27° C dan tekanan 3 ATM? Instant Video.

99.9 5 Kg Nitrogen Gas, 183 Degree C at best price in Ahmedabad ID 22433105655
0.2. US teaspoon. 0.2. About Nitrogen gas. 1 cubic meter of Nitrogen gas weighs 1.251 kilograms [kg] 1 cubic inch of Nitrogen gas weighs 0.000723124 ounce [oz] Nitrogen gas weighs 0.001251 gram per cubic centimeter or 1.251 kilogram per cubic meter, i.e. density of nitrogen gas is equal to 1.251 kg/m³; at 0°C (32°F or 273.15K) at standard.

Jual Tabung Gas Nitrogen Murah , Jual Gas Nitrogen Murah, Jual Tabung Nitrogen Murah, Jual
To determine this value, we rearrange the ideal gas equation to. n V = P RT (10.5.1) (10.5.1) n V = P R T. Density of a gas is generally expressed in g/L (mass over volume). Multiplication of the left and right sides of Equation 10.5.1 10.5.1 by the molar mass in g/mol ( M M) of the gas gives.

99.9 Nitrogen Gas Cylinder, Rs 35 /cubic meter HP Industrial gases ID 23007247488
The mass of 0.560 moles of chlorine gas Cl 2 is 39.71 g. To obtain this value, follow these steps: Determine the molar mass of the gas. Since chlorine has a molar mass of 35.453 g/mol on the periodic table, the molar mass of the chlorine gas Cl 2 is twice this value. This is 70.906 g/mol. Use the molar mass formula to calculate the mass: