
Thí nghiệm *Thử quỳ tím dung dịch NaHSO3 (Sodium bisulfite)* YouTube
NaHSO3 was firstly reacted with aldehyde to form the aldehyde sodium bisulfite, which reacted with o-phenylenediamine to form the 2-substituted benzimidazole and inhibited the formation of 1,2-disubstituted benzimidazole. This protocol solved the poor selectivity problem appearing in traditional method when cyclocondensation between o.
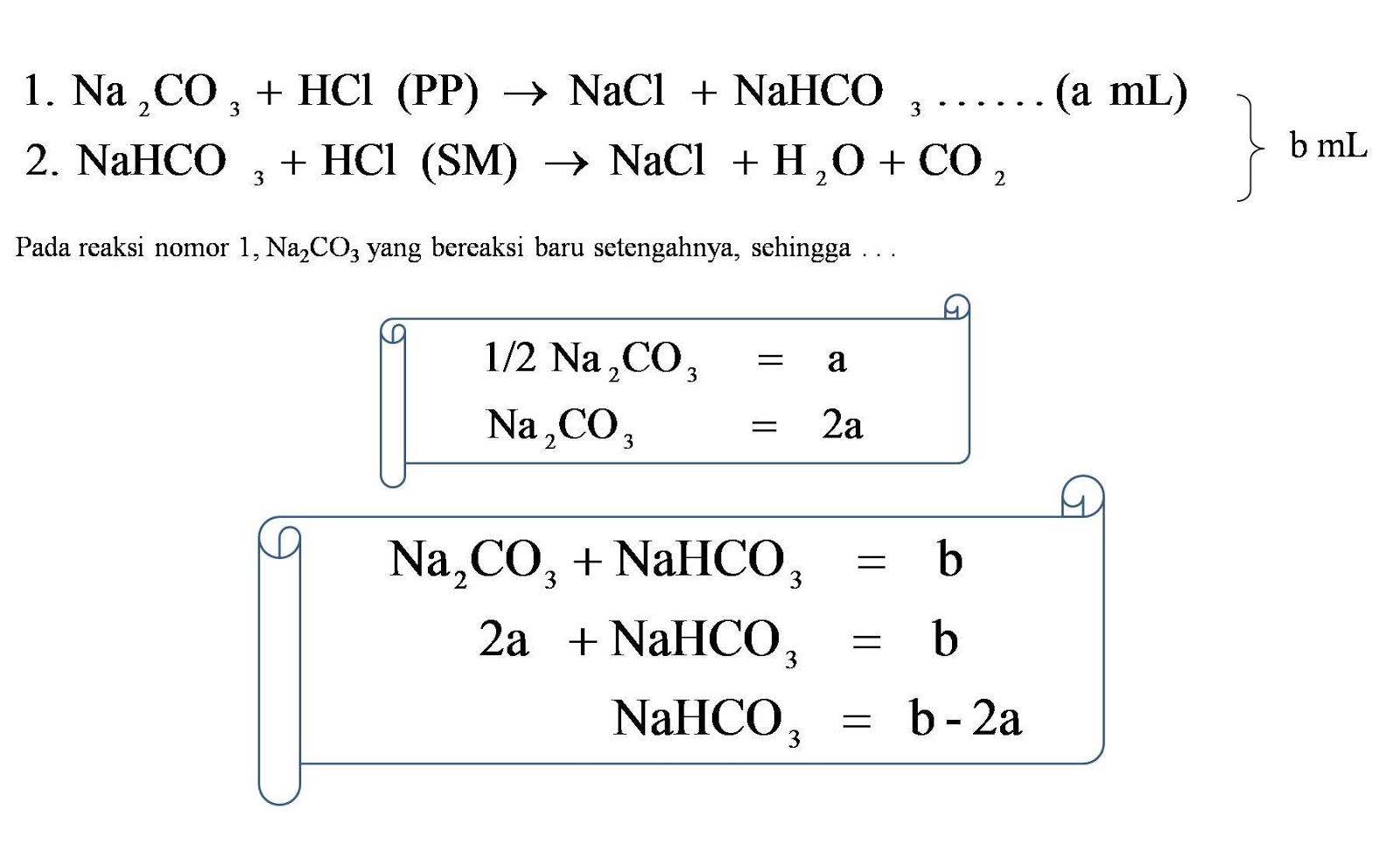
Kadar Campuran Na2CO3 dan NaHCO3 Cara Warder
KOMPAS.com - NaHCO3 atau natrium bikarbonat lebih dikenal dengan nama soda kue. Soda kue atau NaHCO3 termasuk dalam golongan senyawa karena tersusun lebih dari satu jenis unsur.. Unsur penyusun soda kue (NaHCO3) adalah natrium, hidrogen, karbon, dan dan juga oksigen dengan perbandingan 1:1:1:3. Dilansir dari Pubchem, natrium bikarbonat (NaHCO3) adalah bubuk atau gumpalan kristal putih tidak.

NaHSO3(亚硫酸氢钠)溶液中微粒浓度大小比较 哔哩哔哩
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na 2 SO 3.A white, water-soluble solid, it is used commercially as an antioxidant and preservative. It is also suitable for the softening of lignin in the pulping and refining processes of wood and lignocellulosic materials. A heptahydrate is also known but it is less useful because of its greater.

Buy Sodium Bisulfite [NaHSO3] 99+ Food Grade Powder 8 Oz in a Bottle USA Online at desertcartUAE
Word Equation. Sodium Bisulfite + Hydrogen Chloride = Sulfurous Acid + Sodium Chloride. NaHSO3 + HCl = HHSO3 + NaCl is a Double Displacement (Metathesis) reaction where one mole of aqueous Sodium Bisulfite [NaHSO 3] and one mole of aqueous Hydrogen Chloride [HCl] react to form one mole of aqueous Sulfurous Acid [HHSO 3] and one mole of aqueous.
NaHSO3 + NaOH → Na2SO3 + H2O NaHSO3 ra Na2SO3
The following tests are used to identify the presence of aldehydes and ketones. 2,4-dinitrophenylhydrazine test; Sodium bisulphite test; The difference between ketone and aldehyde is the carbonyl group present in aldehydes can be easily oxidised to carboxylic acids, whereas the carbonyl group in ketones is not oxidised easily.This difference in reactivity is the basis for the distinction.

Sodium bisulfite NaHSO3 Trung Quốc hóa chất Việt Quang
Sodium hydrogensulfite is an inorganic sodium salt having hydrogensulfite as the counterion. It has a role as a mutagen and an allergen. It is an inorganic sodium salt and a sulfite salt. It contains a hydrogensulfite. ChEBI. Sodium bisulfite has been used externally for parasitic skin diseases and as a gastrointestinal antiseptic.
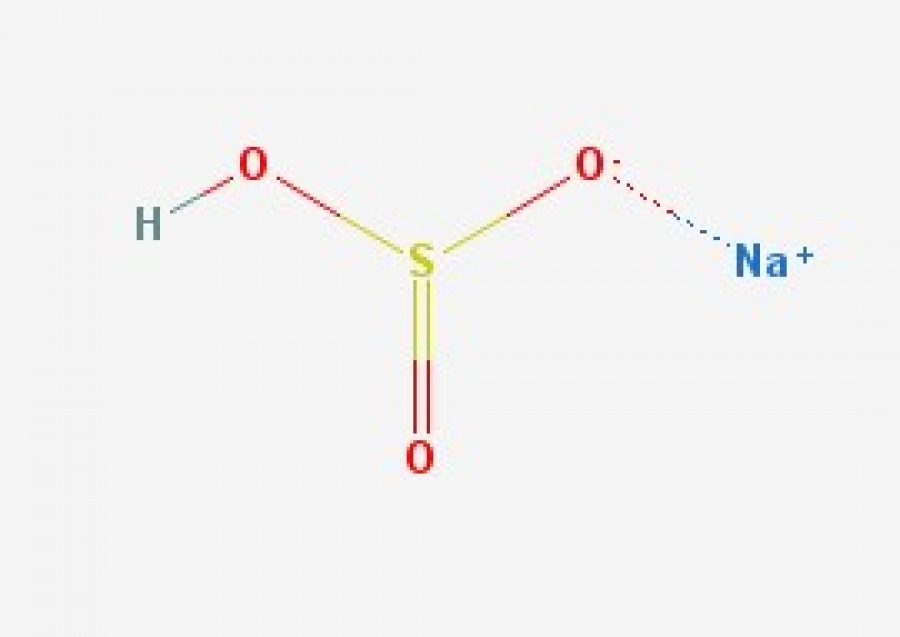
NaHSO3 (Sodium Hydrogensulfite)
NahSO3, juga dikenal sebagai natrium bisulfit, adalah zat kimia yang memiliki banyak kegunaan dalam kehidupan sehari-hari. Dalam artikel ini, kita akan membahas pengertian NahSO3, manfaat dan kegunaannya, serta pengaruhnya pada lingkungan. Apa Itu NahSO3? NahSO3 adalah senyawa kimia yang terdiri dari atom natrium (Na), belerang (S), oksigen (O), dan hidrogen (H). Strukturnya adalah NaHSO3.

Natri bisunfit NaHSO3
Equilibrium reaction relating the two tautomers of bisulfite.. The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO − 3.Salts containing the HSO − 3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na 2 S 2 O 5).Sodium metabisulfite dissolves in water to give a solution of Na + HSO −

Sodium hydrogen sulfite NaHSO3
Since there is an equal number of each element in the reactants and products of NaHSO3 = Na + HSO3, the equation is balanced. Balance NaHSO3 = Na + HSO3 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the.

20) Nucleophilic addition of NaHSO3 in aldehyde ketones Chapter12 class12 neet jee cbse
Chemical Formula: NaHSO3. Melting Point: 150 C. Boiling Point: 315 C. Acidity (pKa): 6.97. Sodium bisulfite (NaHSO3) is a weakly acidic compound that is usually used as a mild reducing agent. Sodium bisulfite is typically used as an aqueous solution. A common application is in the workup of reactions involving bromine or iodine.

NaHSO3 Natri Bisulfit là gì? Ứng dụng, lưu ý sử dụng NaHSO3
Sodium bisulfate, also known as sodium hydrogen sulfate, is the sodium salt of the bisulfate anion, with the molecular formula NaHSO 4.Sodium bisulfate is an acid salt formed by partial neutralization of sulfuric acid by an equivalent of sodium base, typically in the form of either sodium hydroxide (lye) or sodium chloride (table salt). It is a dry granular product that can be safely shipped.

Sodium Hydrosulfite (NaHSO3 35)
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO 3.Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide.

Sodium Bisulfite, Sodium Hydrogen Sulphite, Sodium Hydrogensulfite NaHSO3 Produsen dan Pemasok
NaHSO3 = NaH + SO3 is a redox reaction where S is oxidized and H is reduced. NaHSO 3 is a reducing agent (i.e. it lost electrons) and NaHSO 3 is a oxidizing agent (i.e. it gained electrons). Enter a redox reaction equation to balance it and calculate the reducing and oxidizing agents. Use uppercase for the first character in the element and.

Jual Sodium Hydrogen Sulfite Sodium Bisulfite Natrium Bisulfit Natrium Hidrogen Sulfit
in the reaction of aldehydes with NaHSO3 why will sulphur's lone pair act as nucleophile instead of O-Ask Question Asked 1 year, 4 months ago. Modified 1 year, 4 months ago. Viewed 300 times 0 $\begingroup$ aren't negatively charged species like oxygen anion more nucleophilic than neutral atom like S.

Mechanism of NaHSO3 addition to Carbonyl Compoond of Aldehyde or Ketone Base Catalysed
Natrium bisulfit adalah pepejal anorganik yang terdiri daripada ion natrium Na + dan ion HSO3- bisulfit. Formula kimianya ialah NaHSO3. Ia adalah pepejal kristal putih dan kerana sifat antioksidannya, ia banyak digunakan sebagai pengawet makanan (contohnya dalam beberapa kesesakan)
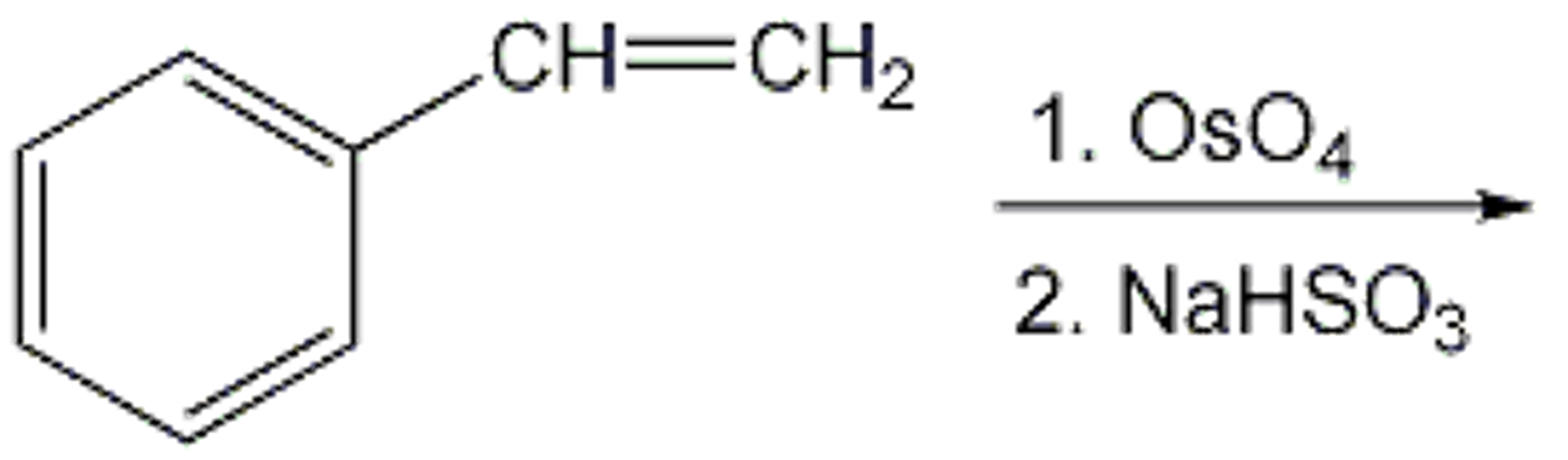
Solved CH=CH2 1.OSO4 2. NaHSO3
Formula : NaHSO3 Molecular weight : 104.06 g/mol Component Classification Concentration sodium hydrogensulphite CAS-No. EC-No. Index-No. 7631-90-5 231-548- 016-064-00-8 Acute Tox. 4; Eye Irrit. 2A; Aquatic Acute 3; H302, H319, H402 <= 100 % For the full text of the H-Statements mentioned in this Section, see Section 16.