
Draw the Lewis dot structure for boron trichloride, BCl_3. Quizlet
Boron trichloride is a starting material for the production of elemental boron. It is also used in the refining of aluminium, magnesium, zinc, and copper alloys to remove nitrides, carbides, and oxides from molten metal. It has been used as a soldering flux for alloys of aluminium, iron, zinc, tungsten, and monel.
BCl3 Lewis Structure (Boron Trichloride) YouTube
BCl3 Lewis Structure, Molecular Geometry, Hybridization and Shape. Written by Priyanka in Science. A trihalide of Boron, BCl 3 consists of a single boron atom and three atoms of Chlorine. It is a colorless inorganic compound that has a pungent odor and appears as fumes in air. It finds use in various applications, including the production of.

BCl3 Lewis Structure in four simple steps What's Insight
Step #1: Calculate the total number of valence electrons. Here, the given molecule is BCl3. In order to draw the lewis structure of BCl3, first of all you have to find the total number of valence electrons present in the BCl3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

So far, we’ve used six of the BCl3 Lewis structure’s total 6 outermost valence shell electrons
The reason why BCl3 lewis structure is more acidic than AlCl3 as the reaction with base 2p orbital of boron in BCl3 lewis structure shows overlap with base. This overlap is much stronger as compared to the 3p orbital overlap of aluminium. ad. Apart from this boron has much more electronegativity than aluminium.

BCl3 Lewis Structure How to Draw the Lewis Structure for BCl3 YouTube
The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons remain, and this lone pair is placed on the Xe atom: Exercise 4.2.2 4.2. 2: interhalogens.

draw the lewis structure for bcl3 in the marvin window below and then decide if the molecule is
3.1: Lewis Structures. Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds.

Bcl3 Lewis Structure / PPT Polar Covalent Bonds; Acids and Bases PowerPoint Mohammad Whitehouse
I quickly take you through how to draw the Lewis Structure of BCl3, (Boron TriChloride). I also go over formal charge, hybridization, shape and bond angle.

BCl3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

Draw a Lewis structure for BCl3 and answer the following questions based on your drawing. 1. For
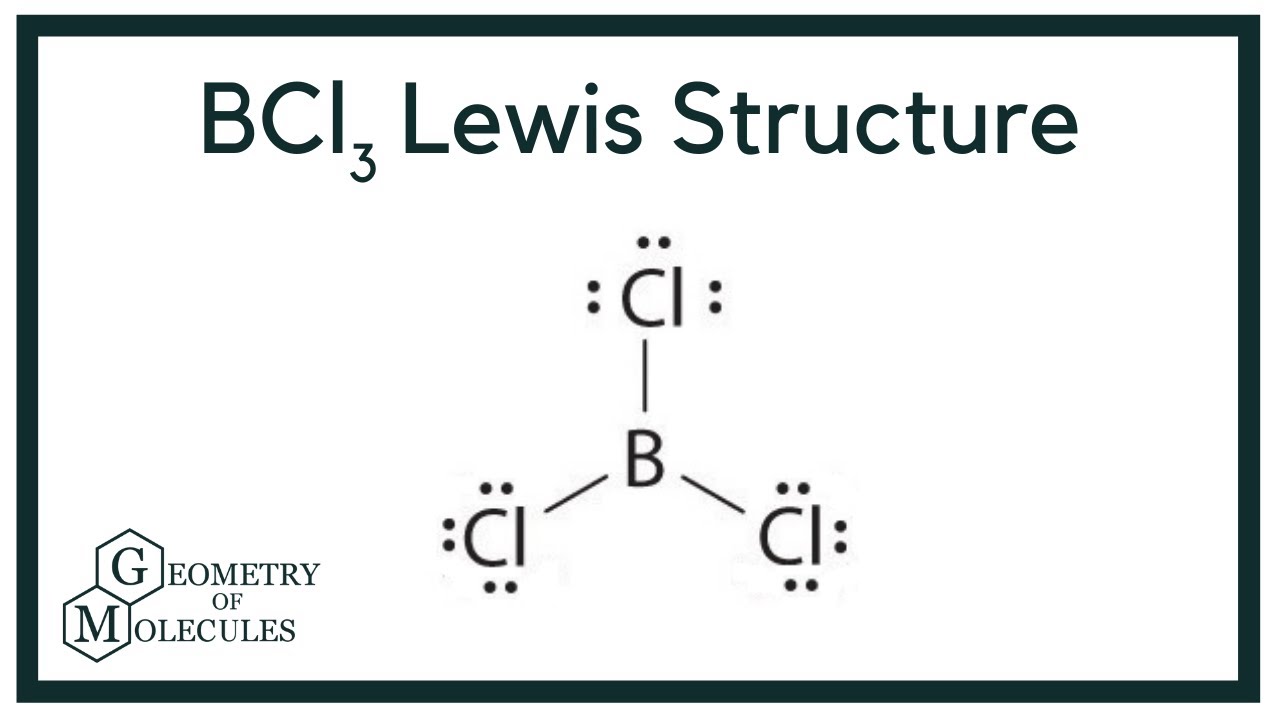
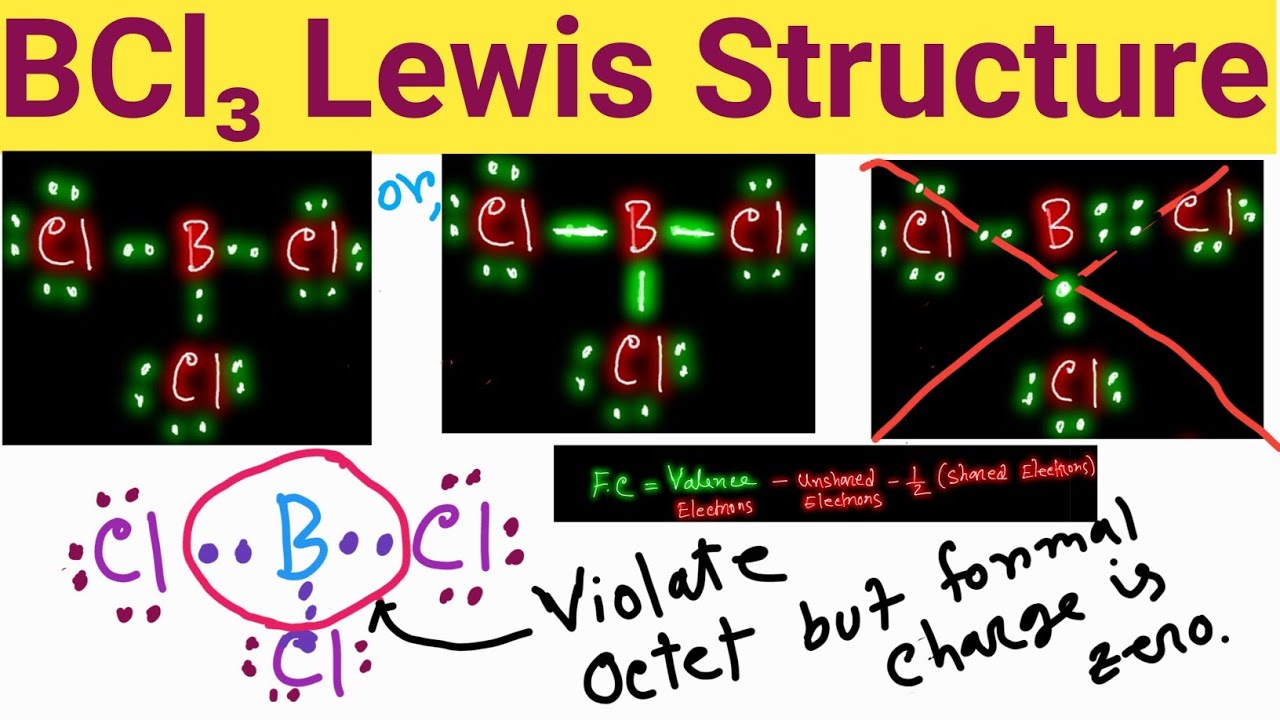
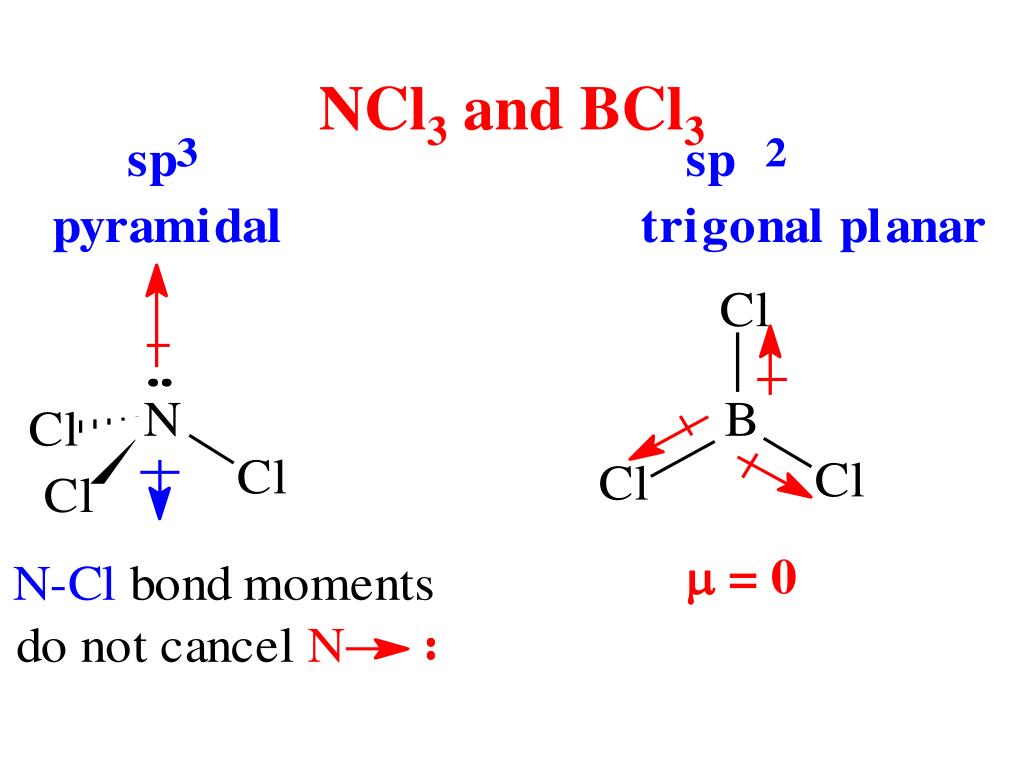
The Lewis structure of BCl3 indicates the following things. 1. Atom arrangement: The Lewis structure of boron trichloride indicates that, the central atom is boron. It is bonded with three Cl atoms and forms trigonal planar structure. Three chlorine atoms are arranged in three vertices of the trigonal. 2.
Lewis Bcl3 Estudiar
To sketch the BCl3 Lewis structure by following these instructions: Step-1: BCl3 Lewis dot Structure by counting valence electrons on the boron atom. Step-2: Lewis Structure of BCl3 for counting valence electrons around the terminal chlorine atom. Step-3: Lewis dot Structure for BCl3 generated from step-1 and step-2.
BCl3 Lewis Structure Lewis Dot Structure for BCl3 Boron Trichloride Lewis Structure YouTube
For the BCl 3 Lewis structure there are a total of 24 valence electrons available. See the Big List of Lewis Structures. Transcript: Hi, this is Dr. B. Let's do the Lewis structure for BCl3. Boron has three valence electrons. Chlorine has 7, but since we have three of those we'll multiply them together. And we get a total of 24 valence electrons.

BCl3 geometry YouTube
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

Draw the Lewis dot structure for boron trichloride, BCl_3. Quizlet
This is free chemistry help for you. This video tutorial will explain how to draw the Lewis dot structure and molecular geometry for boron trichloride (BCl3).

BCl3 lewis structure, molecular geometry, bond angle, hybridization
BCl3 Lewis Structure. Let us apply the lewis dot rules and try to draw the structure of boron trichloride. First of all, we need to calculate the total valence electrons of this molecule, B = 3. C l= 7. 3Cl = 7*3=21. So, total= 21+3= 24. Now, boron is less electronegative, which makes it the central atom.
Lewis Bcl3 Estudiar
Check me out: http://www.chemistnate.com
[Solved] The BCl3 molecule can react with Cl by formation of a coordinate... Course Hero
A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure (Boron trichloride).For the BCl3 structure use the periodic table to find the total nu.