
F2 Lewis Structure / Is F2 Polar Or Non Polar Fluorine Gas Youtube It has a very simple
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.

F2 Lewis Structure
Figure 1.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine. For simple diatomic molecules, combining the Lewis symbols of each element gives its Lewis structure. H2 example: (H only needs two electrons; usually referred to as a duet.) Figure 1.2b The Lewis structure of Hydrogen. F2 example:

Draw the electron dot structure for F2.
The Octet Rule. The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom.This allows each halogen atom to have a noble gas electron configuration. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule.

Struktur Lewis molekul F2 (Z = 9) adalah.
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.
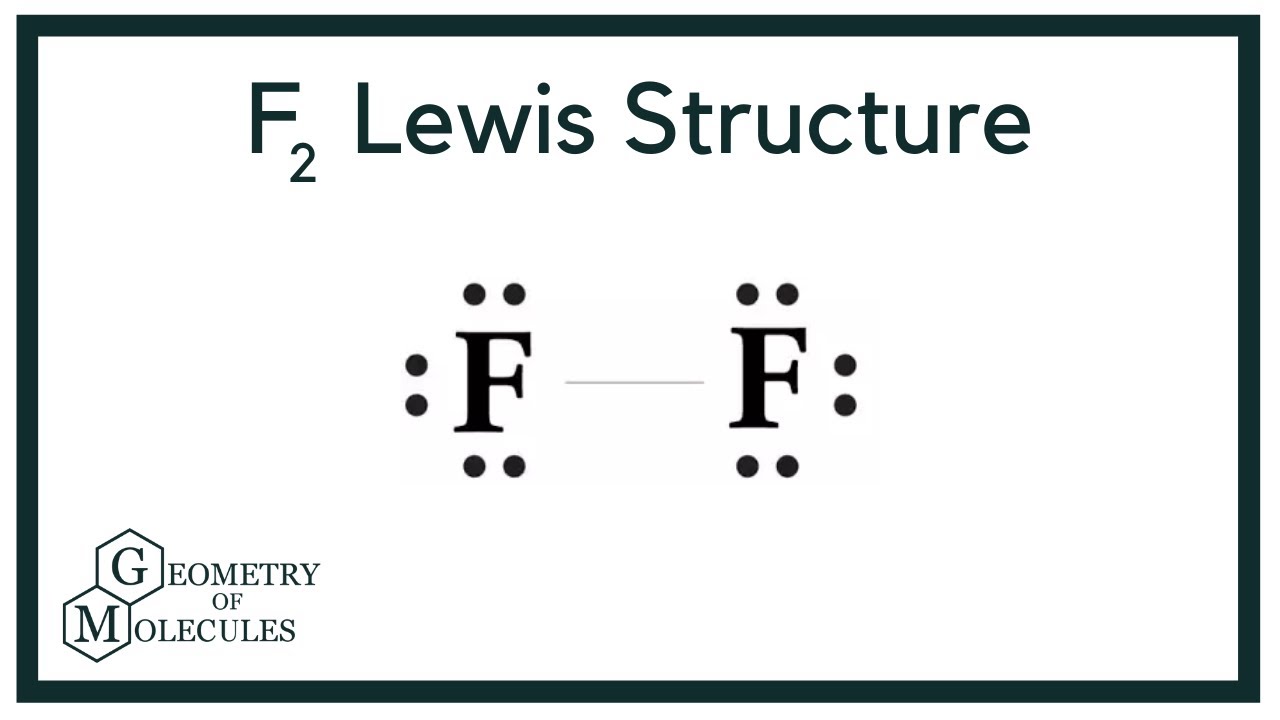
F2 Lewis Structure (Fluorine Gas) YouTube
F2 Lewis Structure A. Definition and concept. The Lewis structure is a representation of a molecule's valence electrons using dots and lines to indicate bonds. For the F2 molecule, there are two fluorine atoms bonded together through a single covalent bond. Each fluorine atom has 7 valence electrons, and they share one electron pair to form.

F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
Step 3: Connect each atoms by putting an electron pair between them. Now in the F2 molecule, you have to put the electron pairs between both the fluorine atoms (F). This indicates that both the fluorine (F) atoms are chemically bonded with each other in a F2 molecule. Step 4: Make the outer atoms stable. Place the remaining valence electrons.

The first step is to put seven valence electrons around the fluorine atom as given in the figure.
Struktur F2 Lewis memiliki dua atom fluor (F) yang mengandung ikatan tunggal di antara keduanya. Ada 3 pasangan elektron bebas pada dua atom fluor (F). Jika Anda tidak memahami apa pun dari gambar struktur Lewis F2 (fluor) di atas, ikuti saja saya dan Anda akan mendapatkan penjelasan langkah demi langkah yang mendetail tentang cara menggambar.
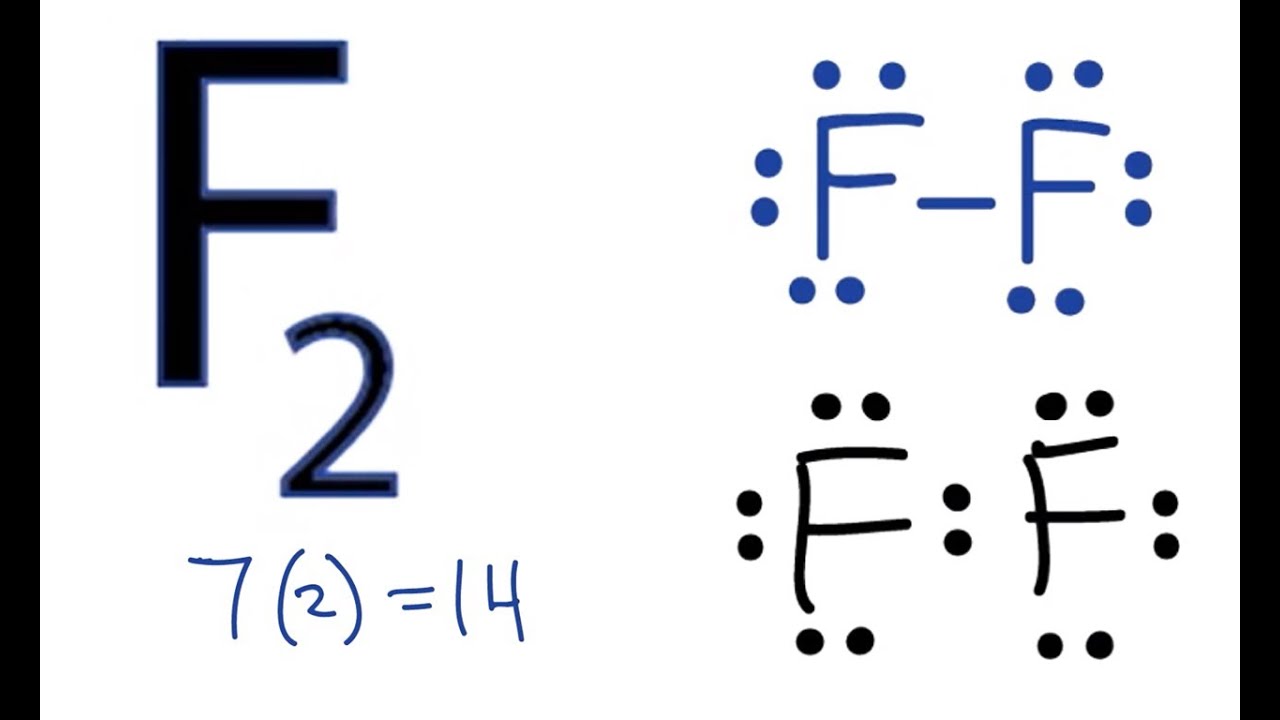
F2 Lewis Structure How to Draw the Lewis Dot Structure for F2 YouTube
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The SiF2 molecule contains a total of 4 bond (s). There are 2 non-H bond (s). Images of the chemical structure of SiF2 are given below: The 2D chemical structure image of SiF2 is also called skeletal formula, which is the.

F2 Lewis Structure
Drawing Lewis Structure of N2F2. Step 1: To draw the Lewis structure of N2F2 we will first have to determine the number of valence electrons in the molecule. Nitrogen is a group 15 element that has 5 valence electrons and requires 3 electrons in order to complete its octet, while Fluorine belongs to group 17 and comprises 7 electrons in its.
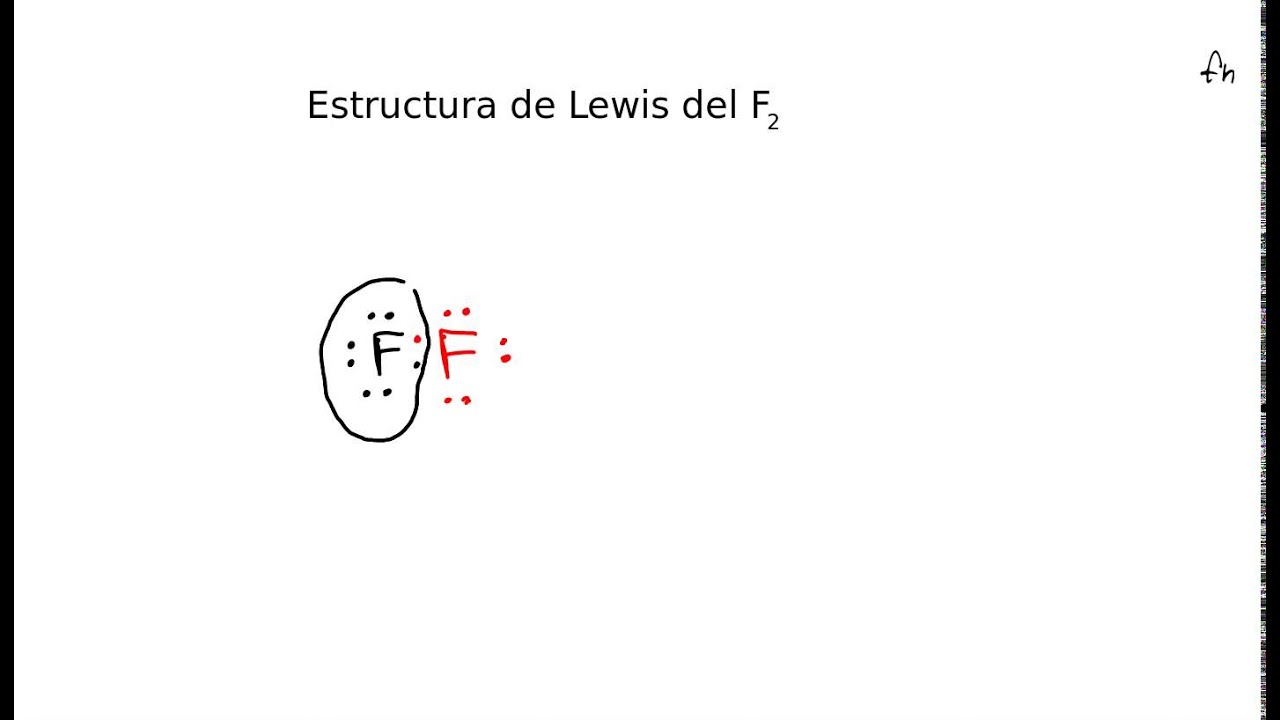
Estructura de Lewis del fluor F2 YouTube
Steps. To properly draw the F 2 Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. Let's break down each step in more detail.

F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.

How to draw F2 Lewis Structure 1
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
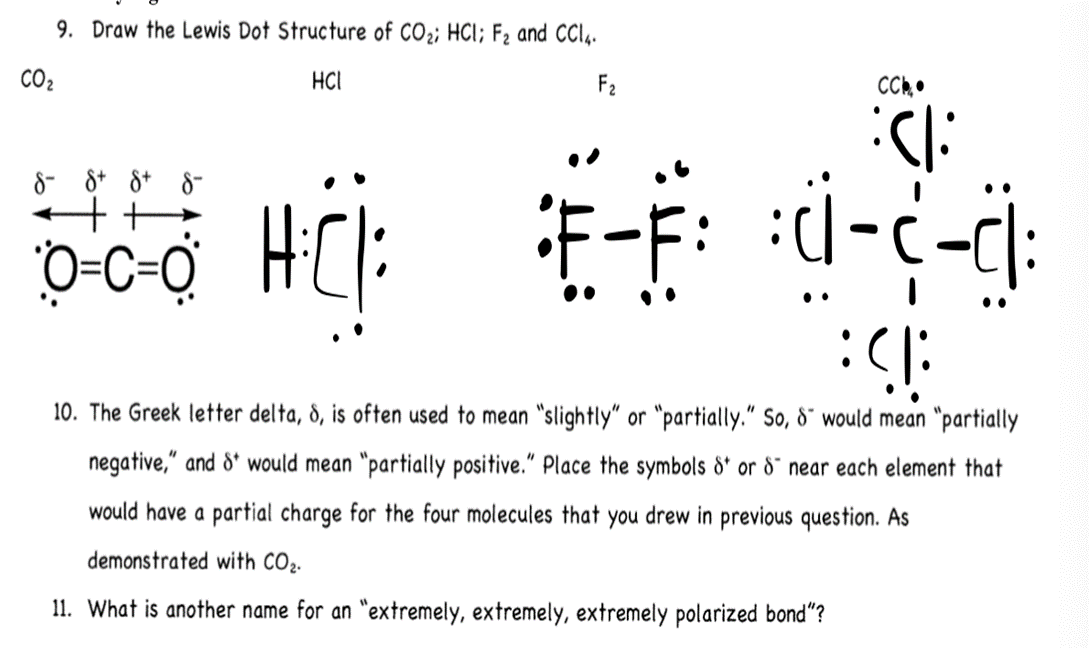
(Get Answer) 9. Draw The Lewis Dot Structure Of CO2; HCI; F2 And CCIA. CO2 HCI... Transtutors
Fluorine, with the chemical formula F2, is a pale yellow-colored diatomic gas, which has a pungent odor. F2 has a molecular weight of 37.997 g/mol. Its boiling point is −188 °C, and its melting point is −219.67 °C. It is toxic in nature; it can cause chemical burns on the skin and can be lethal if inhaled. It is highly reactive, is.

Perhatikan gambar struktur Lewis beberapa senyawa berikut...
Hi Everyone! In this video, we will help you find out the Lewis Structure of Fluorine gas molecules. It has a very simple structure and with our step-by-step.

Draw the electron dot structure of F2?
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Gambarkan struktur Lewis senyawasenyawa berikut!
F2 is a covalently-bonded molecule, with two F atoms single-bonded to each other. There are three lone pairs on each of the F atoms.Check me out: http://www..